/ Investors
Invest in the Future
of Immune Reprogramming
A disruptive, device-enabled platform with multi-indication market potential.

- IRB partnerships in place: Banner, Barrow, Indiana University School of Medicine
- Drug-free, scalable immune-reset therapy
- Applicable across ALS, MS, PD, and Alzheimer’s
- Market Opportunity: $17.9B–$68B (based on TAM analysis)
- IP Portfolio active in US, Japan, Canada, India, Australia
Transforming Neurodegeneration Through Immune Reprogramming
Our lead innovation, SELanetx™, is a point-of-care therapy that uses controlled hyperoxia and mechanotransduction to induce a natural immune reset. With preclinical success, regulatory momentum, and strategic institutional partners, we are poised to redefine the treatment landscape for ALS and beyond.
HOPE-Neuron Therapeutx is a neurobiology company pioneering a non-stem-cell, device-enabled platform to restore immune balance in patients with ALS and related neurodegenerative diseases.
- Primary Focus: Preparing for first-in-human IRB trials with top U.S. institutions, Banner Health.
- Multi-Indication Potential: Core mechanism applicable to ALS, , Parkinson’s, and Alzheimer’s, addressing a $17.9B–$68B market opportunity over 3–10 years.
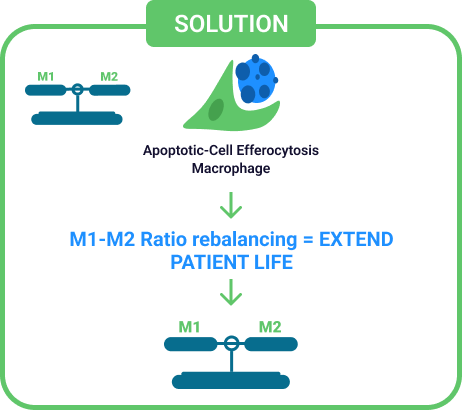
- No Drugs or Stem Cells: 30-minute IV protocol powered by blood-based apoptotic signaling. No immunosuppression. No toxicity.
- Global IP Protection: Active patents granted in the U.S., Canada, Japan, India, and pending in key markets.
- Backed by Data:
Preclinical transgenic mouse study underway with the Indiana University School of Medicine. Results show preserved motor function in ALS mouse models and validated immune shifts (M1 → M2) using biomarkers like TNF-α, CD206, NOS1.
The SELanetx™ Advantage
- Precision Apoptogenics: Triggers beneficial apoptotic cells from a ~300mL blood draw.
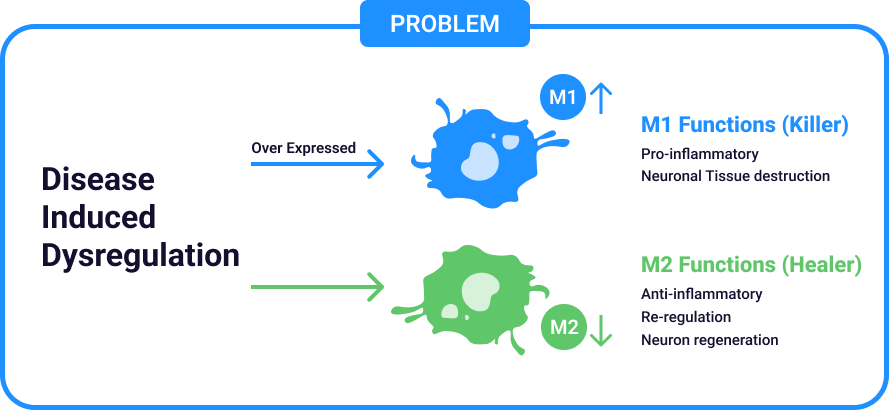
- Efferocytosis in Action: Reprograms chronic inflammatory “killer” cells (M1) into “healer” cells (M2).
- Closed-Loop System: Treatment is sterile, simple, and scalable for real-world clinic use.
"We’re not treating symptoms — we’re addressing the immune root of neurodegeneration."
Active Collaborations
- Gregory W. Fulton ALS Clinic at the Barrow Neurological Institute – Clinical Research Partner
- Banner Health – Clinical research partner
- Indiana University School of Medicine – Preclinical research and biomarker validation
Critical Challenge – Immune
Balance Dysregulation
Immune Plasticity – Dr Jekyll (M2) / Mr. Hyde (M1) Rebalancing
Annual Gross ALS/MS/PD Revenue – 5-10 Years
Ultimate Therapeutic Target: Alzheimer’s Autoimmune-Neurodegenerative Disease, 8-12 Years. SELanetx™ Total Gross Revenue Potential & Market Penetration

HOPE-Neuron Allocation Critical Path Sensitivity
Assumed Prevalence: ALS 34,000 – 2-3 yrs : MS & PD1,000,000 – 5-10 yrs ; Alzheimer’s – 8-12 yrs.
Assumptions: Penetration Sensitivity
Optimistic ALS 45%; MS/PD 15% ea.
Patient # : ALS 15,300; MS/PD 150,000 ea.
Possible ALS 35%; MS/PD 10% ea.
Patient # : ALS 11,900; MS/PD 100,000 ea.
Pessimistic ALS 25%; MS/PD 5% ea.
Patient # : ALS 8,500; MS/PD 50,000 ea.
Patient # : ALS 15,300; MS/PD 150,000 ea.
Possible ALS 35%; MS/PD 10% ea.
Patient # : ALS 11,900; MS/PD 100,000 ea.
Pessimistic ALS 25%; MS/PD 5% ea.
Patient # : ALS 8,500; MS/PD 50,000 ea.
HOPE-Neuron Allocation Critical Path Sensitivity
Provider
35%
Distributor 25%
HOPE-Neuron
40%
M1/M2 Ratio Correlates with ALS Progression
ALS Literature (sample) - M1 toxic to M2 Healing Cells Ratio Correlates with Disease Progression Rates. ALS M1 to M2 Shift Drives Homeostasis and Healing.
Q3 2025
Complete Phase II preclinical studies
Q4 2025
IRB trial site activation
Early 2026
First-in-human dosing
2026+
Expanded indications (MS, PD)
